Almost half of all UK adults regularly take prescription medicines and the 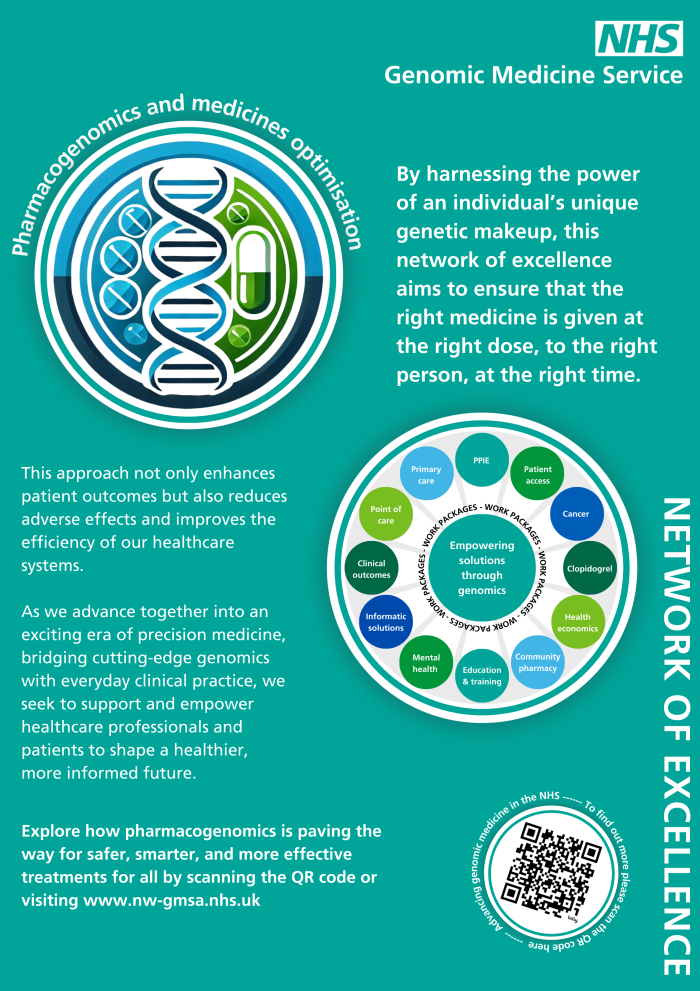 NHS’s annual budget for medicines is approximately £17.4 billion per year, with over 1.1 billion items prescribed annually.
NHS’s annual budget for medicines is approximately £17.4 billion per year, with over 1.1 billion items prescribed annually.
One approach to addressing adverse or ineffective medication reactions is to leverage knowledge of an individual’s genetic information to support medicines optimisation, better informing medicine selection and dosing, a concept known as pharmacogenetics.
This NHS Genomic Network of Excellence will develop the rollout of pharmacogenomics and medicines optimisation in the NHS, including furthering the rollout in primary care.
This NHS Genomic Network of Excellence aligns with the Manchester NIHR BRC, NIHR Academic Health Science Network (AHSNs), industry partners and a Horizon 2020 Grant.
The network has 12 work packages underway. More information avaialble below.
There are 12 work packages underway:
- Progress – An implementation trial of pharmacogenetics in primary care - Prof. Bill Newman
- Progress Rx – Informatics solutions for pharmacogenetics - Scott Watson
- Patient access to pharmacogenetic data – Videha Sharma
- Health economics of pharmacogenetics – Katherine Payne
- Clinical outcomes of introducing pharmacogenetics – John McDermott
- PPIE – Emma Magavern
- Community pharmacogenetics – David Wright
- Cancer pharmacogenetics – Munir Pirmohamed
- Mental health pharmacogenetics – Elvira Bramon
- Point of care pharmacogenetics – Prof. Bill Newman/John McDermott
- Clopidogrel pharmacogenetics implementation – Videha Sharma
- Education and training – Jessica Keen/Emma Groves
Recent webinars
We recently hosted a webinar, on the topic of 'Pharmacogenomics and childhood asthma', hosted by Professor Bill Newman and Somnath Mukhopadhyay, from University Hospitals Sussex NHS Foundation Trust. The recording will be published at 12:00 noon, Tuesday, 16th December 2025.
To watch the recording at that time, please click on the tab shown below.
Media sources
We regularly produce audio visual materials including infographics, animations, newsletters, podcasts and recordings from our national Pharmacogenomics Network of Excellence events.
We also have a case study on the clopidogrel project, entitled 'Overview of the Clopidogrel Pilot Project: CYP2C19 genetic testing for stroke and TIA patients, available to view on our case studies page.
Pharmacogenomics is pivotal in tailoring medical treatments to individuals, particularly because certain adverse drug reactions are determined by genetic factors. Notably, adverse drug reactions contribute to 6.5% of hospital admissions in the UK. Employing pharmacogenomics to assess how individuals react to medications can greatly improve patient safety and treatment outcomes.
In this episode, experts in this field explore the role of genomic testing in determining which patients will benefit from specific medications and which might experience negative effects. They delve into the partnership between Genomics England and the Medicines and Healthcare products Regulatory Agency through the Yellow Card Biobank. The discussion also covers the hurdles in integrating pharmacogenomics into the healthcare system.
The podcast is hosted by Vivienne Parry, who is the Head of Public Engagement at Genomics England, accompanied by Anita Hanson, Research Matron and Lead Research Nurse for clinical pharmacology at Liverpool University Hospitals NHS Foundation Trust, Professor Bill Newman from the Manchester Center for Genomic Medicine, and Professor Matt Brown, the Chief Scientific Officer at Genomics England.
https://www.genomicsengland.co.uk/podcasts/can-genomic-testing-prevent-adverse-drug-reactions.
Professor Bill Newman, project lead for this Genomic Network of Excellence features in a NEW podcast, from Genetic Sounds (July 2024) where they discuss Pharmacogenetics. Recorded with a live audience in Berlin, Germany.
Available to listen by clicking on the image.
Education and training resources
Our Pharmacy Education and Training Resources area provides education and training resources for pharmacists and pharmacy professionals, and signposting to relevant stakeholder organisations.